
Molecular Cations and Anions
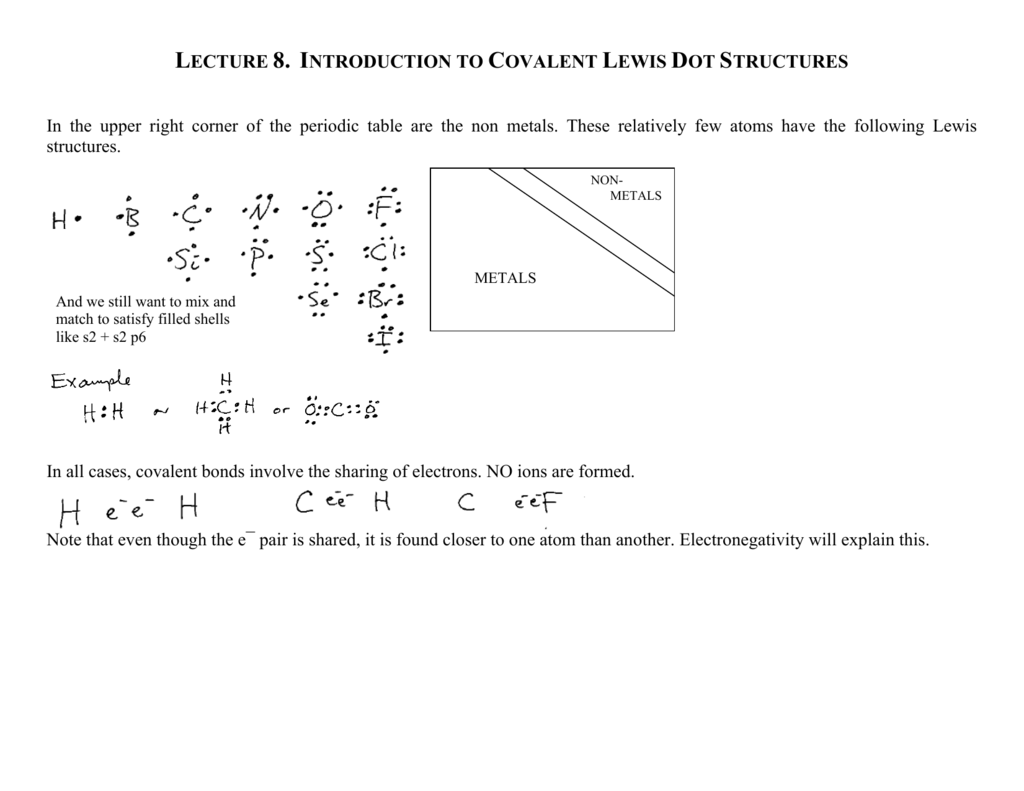
Electrons in the Lewis Dot Structure? ZDraw Lewis Structures for O 2 and N 2. 14 Covalent Bond zThere is an optimum distance between atoms in a covalent bond. This is the bond length: calculated by adding the radii of two atoms. Strengths of Covalent Bonds zCloser nuclei result in a stronger bond. Shorter bond = stronger bond. He described what he called the cubical atom, because a cube has 8 corners, to represent the outer valence shell electrons which can be shared to create a bond. This was his octet rule. Rules for drawing Lewis dot structures. Count the number of valence e-each atom brings into the molecule. For ions, the charge must be taken into account. Here is the simple view on this important topic: In Lewis structures, we show each covalent bond as two dots which represent a pair of electrons. For example: Therefore, we can say that Lewis structures are electron dot representations for molecules. The trick here is to keep track of the electrons and correctly place the atoms in the molecule.
Molecules can also lose or gain electrons to become cations or anions. For example, the NO3 molecule will gain an electron to form the nitrate anion.
If you count up all of the electrons you'll find that all of the atoms feel like neon.
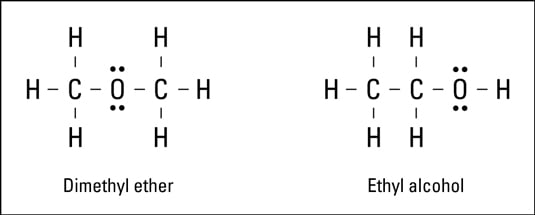
Covalent Bonds Lewis Dot Diagram
Here is the ammonium ion, an example of a molecular cation.
The ammonium ion has given up an electron to become a cation.

Ionic Bonds
Ionic bonds are generally formed when you bring atoms which really want to lose electrons together with atoms which really want to gain electrons.
The Na+ cation and the Cl- anion are held together by electrostatic or ionic bonds. There is no sharing in ionic bonding. The anion takes the electron for itself and the cation is happy to get rid of its electron. The ions in ionic compounds are arranged in three-dimensional structures. There are no discrete molecules of NaCl. We can only write an empirical formula of NaCl.
Lewis Dot Structure Covalent Bonds Calculator Present Value
Here are some other examples.
| NH4Cl : | here NH4+ | = cation |
| here Cl- | = anion | |
| BaCl2 : | here Ba2+ | = cation |
| here Cl- | = anion |
All substances are electrically neutral. We can use this fact to obtain the chemical formula of an ionic compound.
| Ba2+ | and | SO42- | form | BaSO4 |
| Na+ | and | S2- | form | Na2S |
Notice that in Na2S, two sodium cations were needed to balance the -2 charge of S2-, making things electrically neutral.